The Sensitivity of influenza virus group A/HS, isolated from wild birds in Russia, to arbidol in MDCK cell culture
I. T. Fedyakina, I. A. Leneva, S. S. Yamnikova, D. K. L’vov, R. G. Glushkov, A. M. Shuster
Virology Institute of D. I. Ivanovsky, RAMN
Scientific Research Chemical-Pharmaceutical Institute
“Masterlik” Company, Moscow
Summary: The effect of the antiviral drug arbidol on the reproduction of avian influenza A/H5 viruses was studied in in vitro experiments. The strains were isolated from the wild birds of Eastern Siberia and they were closely related to the 1997-2000 viruses from Southeast Asia. Arbidol was shown to exert a selective inhibiting effect on the reproduction of these viruses in MDCK cell cultures.
Key words: avian influenza A virus, arbidol, virus-inhibiting activity
Group A influenza viruses infect various species of birds and mammals, including humans, causing pandemics and epizootics. The natural hosts of influenza viruses are certain bird species of aquatic and near-water environments. Influenza viruses are characterized by highly heterogeneous combinations of the surface proteins hemagglutinin (HA) and neuraminidase (NA) and are represented, according to nomenclature, by 16 subtypes of HA and 9 of NA [10]. Viruses including all the known combinations of surface proteins have been isolated from wild birds.
Despite the heterogeneity of the influenza viruses antigen, however, only three subtypes of HA (H1-H3) and two of NA (N1-N2) circulate among humans. Periodically, an infection of avian flu is noted among humans or other mammals. The outbreak of flu cases among people in Hong Kong in 1997 was the first sign of the bridging of a cross-species barrier between birds and humans under natural conditions. That was the first case of direct transmission of the “bird flu” influenza viruses A/H5N1 to humans, at which time out of 18 infected people, six died. The transmission of the virus from person to person was not explained [9, 11, 16]. In October 2003, in Vietnam and China, 14 people (13 children and 1 adult) were infected with the H5N1 virus, and 12 died. During 2004, the number of infected people in various Asian countries grew. By the beginning of 2005, there had been registered 45 fatal cases of “bird flu.”
The rise of a correlation between highly pathogenic viruses in birds and a human influenza epidemic raises a real threat of new pandemic viruses. Up to the present time, there has not been a vaccine against this serotype, since the standard method of preparation of the flu vaccine with chicken embryos is made difficult due to the pathogenicity of these viruses to the embryos.
All of the above shows the pressing need to develop and create preparations which can be approved for use against influenza.
The widespread use in Russia of the preparation “arbidol” to prevent and treat influenza group A as well as B [1, 4], and the lack of data concerning arbidol’s effects in relation to influenza viruses A/H5, which appears to be a real threat at the present time, provided the foundation for the study of the effect of this preparation on these viruses.
Materials and Methods
Viruses and cells. influenza viruses A/H5N2 and A/H5N3, isolated from wild birds migrating from China, were used in this study. The isolates have a specific HA structure, closely related in primary structure to the viruses which caused the outbreak of disease in humans and poultry in the countries of Southeast Asia [6, 15]. The viruses were cultivated in the allantoic cavity of 10-day-old chick embryos. MDCK cells were cultivated in 96- and 24-well plates from Costar (USA), in MEM with 10% fetal calf serum (Gibco, USA), 10 mM glutamine and antibiotics.
Preparations. In the experiments the following preparations were used: arbidol, made by the company “Masterlik”; rimantadine (by “Adamantan,” Moscow) and virazol (ICN, USA).
Cytotoxic action (IC50) was determined, as described earlier [3].
Antiviral activity of the preparations under study in relation to influenza viruses A/H5 was assessed according to the lowering of the viral titer in the MDCK cell culture and the level of viral antigens as tested by immunoenzyme assay.
Before inoculation, the MDCK cells were washed twice in medium without serum, to reduce the possibility of nonspecific reactions. Then the preparations were added in the specified concentration in 100 mcl MEM media and incubated with the cells for 1.5 hours. The infection was conducted with 10 cultivations of the viruses on MEM medium with added trypsin (TPCK treated, “Sigma,” USA) in the concentration of 2 mcg/ml. The absorption of the virus was conducted during a 40-minute period, at 37 degrees Celsius. The unabsorbed virus was removed by a 3 rinses in medium without serum. Then varying concentrations of the preparations under study were added to the single layer of cells in the MEM medium containing 2 mcg/ml of trypsin. The control viruses and cells were grown in the same medium. Further, the plates were incubated in a thermostatic environment with CO2 for 72 hours at 37 degrees Celsius. The result assessment was done at the end of 72 hours.
For the study of the effectiveness of the antiviral preparations using the immunoenzyme assay method, MDCK cells were cultured in 96-well plates. Before exposure to the virus, the MDCK cells were washed twice in medium without serum, to reduce the possibility of nonspecific reactions. The preparations were added to the cells in two-stage concentrations in 100 mcl MEM media. 100 mcl of the media was added to the control virus, and 200 mcl was added to the cell control. After the incubation of the cells with the preparations under study for 1.5 – 2 hours at 37 C, 100 mcl of the virus was added to the MEM media on the plates, except for the control cell plate. The level of infection ranged from 0.1 – 0.01 TCID50 per cell. All of the procedures were done in MEM media with added trypsin in the concentration 2 mcg/ml. Then the plates were incubated with CO2 for 20 hours at 37 degrees Celsius. After incubation the cells were examined under an inverted microscope, in order to record the absence of any cytotoxic or cytopathic changes. The medium was removed and the cells were fixed with 80% acetone in a phosphate buffer saline (PBS) for 15 min., were thoroughly dried, and then rinsed three times with a PBS solution containing 0.05% twin-20. These and all following procedures were conducted using this described solution. Then we added to the cells 100 mcl of a solution of PBS with 1% fetal serum and 0.05% twin-20, and incubated at 37 degrees Celsius for 30 min. After removal of the solution, we added to the cells 100 mcl monoclonal antibodies to NP-proteins of influenza A in the concentration 10 mcg/ml. After incubation with the antibodies for one hour at 37 C, we introduced 100 mcl rabbit IgG against mouse IgG, marked with a horseradish peroxidase in 1:1000 cultivation. After 4 rinses, the remaining peroxidase was revealed with the addition of 100 mcl tetramethylbenzidine substrate. The plates were read at 450 nm according to optical density (OD) units with a spectrophotometer (“Biokom,” Russia). Every cultivation of the virus was analyzed 4 times, from which was taken the mean OD450. Inhibition rate was determined by the following formula: [OD450(experiment) – OD450(cell control)] / [OD450(virus control) – OD450(cell control)] X 100%.
Table 1 – Influence of arbidol on reproduction of various strains
of influenza viruses A/H5 in MDCK cell culture
|
Influenza Viruses |
Virus titer, lg TCID50/ml |
|||
|
virus control |
rimantadine 5 mcg/ml |
virazol 5 mcg/ml |
arbidol 10 mcg/ml |
|
|
A/duck/Altai/1285/91 - H5N3 |
8.0 |
6.0 |
6.0 |
5.5 |
|
A/duck/primer/2633/01-H5N3 |
7.0 |
5.0 |
5.0 |
5.0 |
|
A/duck/primer/2621/01-H5N2 |
3.0 |
0.5 |
0.5 |
1.0 |
Note: Above are the results of three identical experiments.
Results
Determination of IC50 showed that that for arbidol it was 40 mcg/ml, for rimantadine, 60 mcg/ml, and for virazol – 100 mcg/ml.
The study of the antiviral effectiveness of arbidol. In the first series of experiments the effect of arbidol was studied in comparison with that of rimantadine and virazol on the reproduction of avian influenza virus A/H5 in MDCK cell cultures. From Table 1 it follows, that arbidol in the concentration 10 mcg/ml, similarly to rimantadine and virazol in concentrations of 5 mcg/ml, effectively inhibits all 3 types of bird influenza virus A/H5. The inhibitory action of these preparations was made clear by the decrease of the influenza viruses infection titer by an average of 2-2.5 lg TCID50/ml in relation to all of the studied A/H5 flu types.
Further, we studied the effectiveness of varying concentrations of arbidol in relation to similar concentrations of rimantadine and virazol on the reproduction of influenza A/H5 with immunoenzyme assay testing on identical levels of infection. With the goal of explaining the specifics of the drug’s action on influenza viruses with different HA antigen structures, in experiments with the same conditions, we determined arbidol’s effectiveness in relation to the usual laboratory strains of influenza viruses, including the subtypes earlier circulating among humans: A/PR/8/34 (H1N1) and A/Aichi/68 (H3N2).
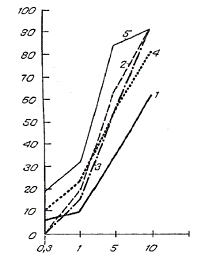
The influence of various concentrations of arbidol on the reproduction of influenza viruses A in humans and birds in MDCK culture. Along the ordinate – percent of lowering of OD450; along the abscissa – concentration of arbidol (in mcg/ml).
1 – A/duck/primer/2633/01 (H5N3)
2 – A/duck/primer/2621/01 (H5N2)
3 -- A/duck/Altai/1285/91 (H5N3)
4 – A/PR/8/34 (H1N1)
5 – A/Aichi/68 (H3N2)
The data on the influence of various concentrations of arbidol on avian influenza viruses reproduction, shown in the figure, characterize the drug’s effectiveness against viral reproduction as dependent on drug concentration. Arbidol inhibits the reproduction of influenza viruses A/H5, and its inhibitory effect is directly proportional to its concentration. At 1 mcg/ml it insignificantly reduced reproduction, as shown by the OD450 size. At a dose of 5 mcg/ml, arbidol’s suppressive effect on the OD450 is heightened; and at 10 mcg/ml, the numbers are 62%, 92% and 91% respectively against influenza viruses A/duck/primer/2633/01 (H5N3), A/duck/primer/2621/01 (H5N2), and A/duck/Altai/1285/91 (H5N3).
Experiments on varying concentrations of rimantadine and virazol produced analogous graphs showing the dependence of viral inhibitory effect on drug concentration. The concentrations of these preparations, inhibiting viral reproduction by 50%, are shown in Table 2. The MIC50 (minimal inhibitory concentration) for arbidol is 4.4, 3.4 and 7.5 mcg/ml against influenza viruses A/duck/Altai/1285/91 (H5N3), A/duck/primer/2621/01 (H5N2), and A/duck/primer/2633/01 (H5N3) respectively. For rimantadine and virazol, the MIC50 values are lower than for arbidol: in relation to influenza viruses A/duck/Altai/1285/91 (H5N3) they are 0.9 and 1.5mcg/ml; A/duck/primer/2621/01 (H5N2) – 0.7 and 1 mcg/ml; and A/duck/primer/2633/01 (H5N3) – 0.4 and 3 mcg/ml respectively.
Table 2 – Minimal inhibiting concentration (MIC50) for various antiviral preparations in relation to different strains of influenza viruses A/H5
|
Influenza Viruses |
MIC50, mcg/ml |
||
|
ribavirin |
rimantadine |
arbidol |
|
|
A/duck/Altai/1285/91 - H5N3 |
1.5 |
0.9 |
4.4 |
|
A/duck/primer/2633/01-H5N3 |
3.0 |
0.4 |
7.5 |
|
A/duck/primer/2621/01-H5N2 |
0.9 |
0.7 |
3.4 |
Discussion
This series of experiments demonstrated that arbidol, rimantadine and virazol, in MDCK cell cultures, in doses nontoxic to cells, effectively inhibit reproduction of avian influenza viruses A/H5, isolated from wild birds on Russian territory. The drugs’ effectiveness increased with an increase in their concentrations. Arbidol displayed a sufficiently high inhibitory effect both in relation to avian influenza viruses A/H5, and human influenza viruses.
Comparative studies of virus-inhibiting concentrations showed that the MIC50 for arbidol was higher than for ribavirin and rimantadine. However, if the expression of MIC50 takes into consideration molar concentrations, then keeping in mind that the molecular weights of virazol and rimantadine are two times lower than arbidol, the difference in MIC50 becomes smaller.
In studying the mechanism of the action of arbidol, the authors have shown that the virus-specific target of its effect on the cycle of viral reproduction is influenza viruses HA. Arbidol interacts with influenza viruses HA to increase its stability against conformational changes induced by a lowering of pH, and as a result inhibits the fusion of the virus’ lipid membrane with the endosomal membrane, which would lead to release of the virus nucleocapsid and the start of virus genome transcription [1, 2, 4, 14].
The results of our study effectively showed the virus-specific action of arbidol on the HA subtypes H1N1, H3N2, H5N2, and H5N3. In addition, our results point to the strain-specific action against influenza of arbidol, rimantadine and virazol in MDCK cell cultures in relation to influenza viruses A/H5. Specifically, viral reproduction of A/duck/Altai/1285/91 (H5N3), structurally the closest to the dangerous viruses causing the outbreak of illness among people and birds in Southeast Asia and Europe (1997 – 2003), was inhibited by 2.5 lg TCID50/ml. Arbidol and virazol inhibited reproduction of H5 influenza viruses, A/duck/primer/2633/01 (H5N3) slightly more weakly in relation to other isolates from primer. However, this virus, according to our data, like virus A/duck/Altai/1285/91 (H5N3), caused disease in mice without prior adaptations (the data is not presented here).
The study of several strains of A/H5, which were differentiated by receptor site structure and gene content [6, 15], showed that the differentiation of receptor action in influenza viruses A/H5 had no significant influence on the effectiveness of arbidol in relation to these viruses.
The WHO, in its campaign against the so-called “bird flu,” has recommended the use of anti-influenza preparations. It is supposed that in the first stage of a possible pandemic, virus-specific chemical preparations will be the primary means of fighting this campaign. However, a seriously limiting factor in the use of adamantadine-type drugs has been the rapid build-up of resistance to them [8]. The H5N1 viruses, isolated from infected people in 2003-2004 in Southeast Asian countries, have already proved resistant. This would be particularly dangerous under the conditions of a pandemic caused by a highly pathogenic virus [7].
At present the effectiveness of the use of NA inhibitors in influenza has been shown [12, 13], but there is no analogous preparation in Russia, and the cost of importing these drugs is relatively high. In addition, according to WHO data, the existing facilities for producing and providing these drugs are relatively small, and for countries which do not produce them, they are not practically obtainable. Therefore, cost and availability will be deciding factors in the choice of antiviral drugs, along with relative effectiveness against influenza viruses. Widely used in Russia, arbidol provides a reasonable solution to these demands.
BIBLIOGRAPHY
- Glushkov R. G., Fadeeva N. I., Leneva I. A. et al. Molecular-biological characteristics in the action of arbidol– New antiviral drugs//Chemical-Pharm. J., 1992, No. 2, pp. 8-15.
- Gus’kova T. A., Glushkov R. G. Arbidol – Immunomodulator, interferon inducer, antioxidant//M., 1999.
- Leneva I. A., Fadeeva N. I., Fedyakina I. T. et al. Looking at virus-specific immunoenzyme indications in the study of the new antiviral drug arbidol// Chem.-Pharm. J., 1994, No. 9, pp. 4-8.
- Leneva I. A., Glushkov R. G., Gus’kova T. A. Medicinal preparations for chemical treatment and chemical prophylaxis of influenza: specifics of mechanism of action, safety and effectiveness Chem.-Pharm. J., 2004, Vol. 38, No. 11, pp. 8-14.
- L’vov D. K., Zhdanov V. M. Persistent genes in viral epidemics and natural populations //Questions in Virology, 1982, No., 4, pp. 17-20.
- L’vov D. K., Yamnikova S. S., Fedyakina I. T. et al. The ecology and evolution of the influenza virus in Russia (1979-2002g.)// Questions in Virology, 2004, No. 3., pp. 17-24.
- Avian Influenza: Assessing the pandemic threat//WHO Report, January 2005.
- Belshe R. B., Hay A. J. Drug resistance and mechanisms of action on influenza A viruses//J. Resp. Dis., 1989, Vol. 10, pp. 952-961.
- Cauthen A. N., Swayne D. E., Schultz-Cherry S. et al. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans//J. Virol., 2000, Vol. 74, No. 14, pp. 6592-6599.
- Foucher Ron A. M., Munster Vincent, Wallenstein Anders et al. Characterization of a novel A influenza virus hemagglutinin subtype (H16) obtained from black-headed gulls//J. Virol., 2005, Vol. 79, No. 5, pp. 2814-2822.
- Ito Toshihiro, Goto Hideo, Yamamoto Eiji et al. Generation of a highly pathogenic avian influenza A virus from an avirulent field isolate by passaging in chickens//J. Virol., 2001, Vol. 75, No. 9, pp. 4439-4443.
- Leneva I. , Roberts N., Govorkova E. et al. The neuraminidase inhibitor GS4014 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza virus//Antiviral Res., 2000, Vol. 48, No. 2, pp. 101-115.
- Leneva I., Golubeva O., Fenion R. et al. Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals//Antimicro. Agents Chemother., 2001, Vol. 45, No. 4, pp. 1216-1224.
- Leneva I., Hay A. The mechanism of action of arbidol against influenza virus-selection and characterization of arbidol-resistant mutants//12th International Congress of Virology, 2002, Paris, Abstr. 1077.
- L’vov D. K., Yamnikova S. S., Fedyakina I. T. et al. Evolution of H4, H5 influenza viruses in natural ecosystems in Northern Eurasia (2000-2002)//Proceedings of the International Conference on Options for the Control of Influenza V. Okinawa, Japan, 7-11 October 2003. International Congress Series, June 2004,Vol. 1263, pp. 169-173.
- Subbaro K., Klimov A., Katz J. et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness//Science, 1998, Vol. 279, pp. 393-396.